Key Takeaways
- Researchers at Science Tokyo have developed a versatile spinel-type sulfide semiconductor, (Zn,Mg)Sc2S4, capable of emitting light from violet to orange at room temperature.
- This new material can be chemically tuned for p-type and n-type conduction, paving the way for efficient LEDs and solar cells.
- The research addresses the “green gap” issue in LED efficiency, making it a promising candidate for next-generation optoelectronic devices.
Innovative Semiconductor Development
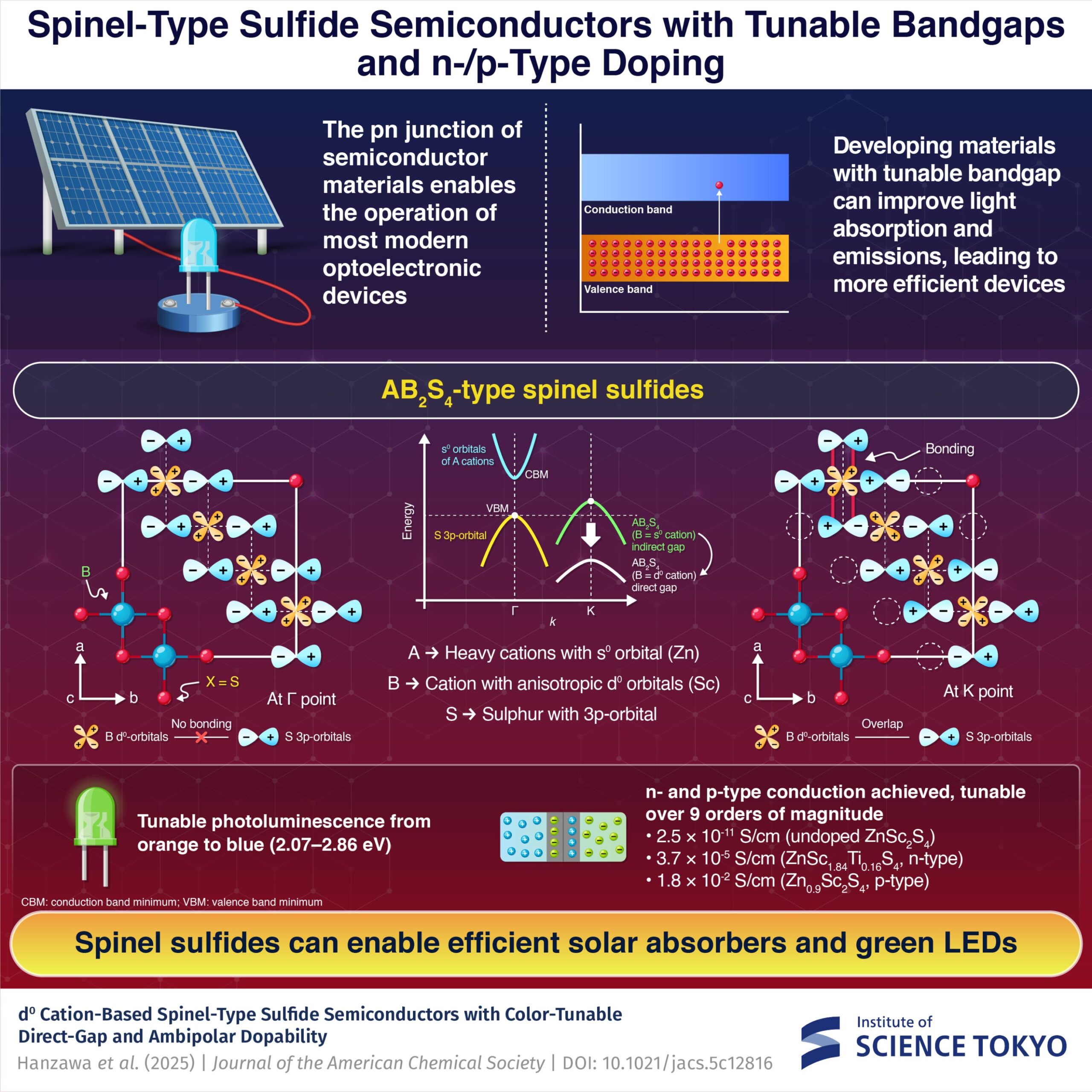
Researchers at the Institute of Science Tokyo have created a groundbreaking spinel-type sulfide semiconductor known as (Zn,Mg)Sc2S4. This material is capable of emitting a spectrum of colors from violet to orange at room temperature, overcoming many efficiency limitations associated with current LED and solar cell technologies.
The innovation stems from the ability to chemically adjust (Zn,Mg)Sc2S4 to switch between n-type and p-type conduction. This flexibility positions the semiconductor as an ideal candidate for creating next-generation pn homojunction devices essential for efficient LEDs and solar cells.
LEDs, solar cells, and semiconductor lasers operate through pn junctions, where an electron-rich n-type region meets a hole-rich p-type region. At these junctions, carriers either recombine to emit light or are separated to produce current. While traditional materials like gallium arsenide (GaAs) excel at efficient light emission, silicon, although superior in capturing sunlight, performs poorly as a light emitter. Thus, finding new materials for improved efficiencies remains an ongoing challenge for researchers.
Led by Professor Hidenori Hiramatsu and Associate Professor Kota Hanzawa, the research team published their findings in the Journal of the American Chemical Society on September 17, 2025. They highlight how their newly developed semiconductor is specifically suited for addressing the green gap problem – a dilemma in LED technology where efficiency drops in the green light spectrum.
Spinel-type sulfides are structured with heavy cations at the A site and lighter cations at the B site, which influences their electronic properties. In (Zn,Mg)Sc2S4, the conduction band minimum is linked to the s orbitals of zinc, while the valence band maximum comes from the sulfur atoms. This arrangement results in a stable band structure and a direct bandgap, critical for effective light emission.
Undoped ZnSc2S4 demonstrates strong orange emission at room temperature. The introduction of magnesium can adjust this emission color from orange to green and even blue. Further modifications, such as adding small amounts of titanium or slightly reducing zinc levels, enable the material to switch between n-type and p-type conduction.
This versatility allows for significant modulation of conductivity, which can vary from the insulating state of undoped ZnSc2S4 to conductive levels in other formulations. These properties make the semiconductor valuable in both solar cell absorption layers and as green-emission layers in LEDs.
“Our semiconductor material is suitable for both green emission and photovoltaic applications,” states Hiramatsu, underscoring its potential in next-generation optoelectronic devices. The advancements in (Zn,Mg)Sc2S4 represent a significant step toward more efficient and versatile materials, possibly transforming the landscape of LED and solar technologies.
The content above is a summary. For more details, see the source article.