Key Takeaways
- New guidelines are being proposed in the EU for harmonizing the assessment and reimbursement of digital medical devices.
- Mutual learning between member states is highlighted as essential for effective integration into healthcare systems.
- A focus on real-world evidence and patient-centered evaluation frameworks is seen as crucial for advancing digital health initiatives.
Introduction to Harmonization Efforts
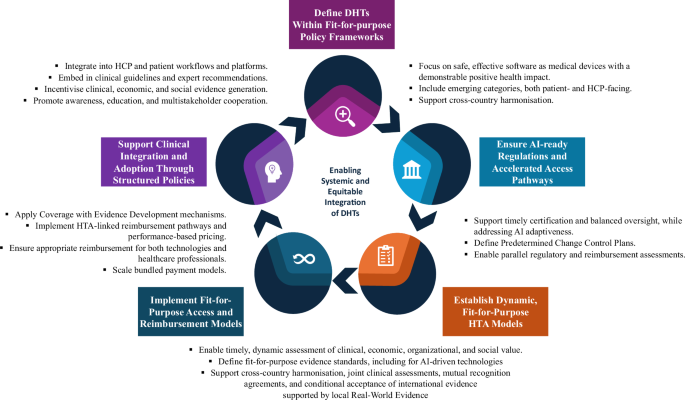
The European Union (EU) is taking significant steps toward harmonizing the assessment and reimbursement of digital medical devices. This initiative aims to simplify processes across member states while ensuring that digital health solutions are effectively integrated into existing healthcare systems.
Importance of Mutual Learning
To achieve these goals, the EU emphasizes the importance of mutual learning among member states. Collaborative efforts can lead to streamlined regulations and enhanced understanding of successful implementation strategies. Focus areas include engaging stakeholders in real-time evaluations of digital health technologies to better assess their effectiveness and cost-efficiency.
Focus on Real-World Evidence
A key component of these reforms is the emphasis on real-world evidence (RWE). By bridging the gap between clinical trials and actual patient experiences, RWE can provide vital insights into the performance of digital medical devices in everyday settings. This approach requires investment in robust data collection and analysis frameworks to ensure that devices are addressing patient needs effectively.
Framework for Patient-Centered Evaluation
Establishing a patient-centered evaluation framework is deemed critical for the successful deployment of digital health technologies. This framework will prioritize patient outcomes and ensure that innovations genuinely meet the needs of end-users. Stakeholders are encouraged to align their assessments with these patient-centered objectives to foster trust and reliability within healthcare systems.
Future Directions
The EU’s ongoing regulations and initiatives, including the Digital Decade Policy Programme and the Health Technology Assessment regulation, aim to reinforce these frameworks. By 2030, the EU seeks to create comprehensive pathways that facilitate the approval and reimbursement of digital health technologies, ultimately ensuring greater access for patients.
In conclusion, the EU’s coordinated approach to harmonizing assessment and reimbursement processes for digital medical devices is crucial for enhancing the adoption of these technologies. Fostering mutual learning, emphasizing real-world evidence, and centering evaluations around patient needs are pivotal strategies that will shape the future landscape of digital health in Europe.
The content above is a summary. For more details, see the source article.